√無料でダウンロード! heterogeneous catalysis and homogeneous catalyst 686662-What is homogeneous catalyst with example
In homogeneous catalysis, reactivity arises from molecular catalysts in a homogeneous solution, while in heterogeneous catalysis reactivity comes from sites on a surface The main advantage of homogeneous catalysis is the ability to design reaction sites within molecularly defined catalysts in order to achieve high catalytic activity, measured Heterogeneous CatalysisHeterogeneous Catalysis Desorption (STEP 4) There is a rearrangement of electrons and the products are then released from the active sites Adsorption (STEP 1) Incoming species lands on an active site and forms bonds with the catalyst 114 Hydrogenation with Homogeneous Catalysts Hydrogen addition to multiple bonds is catalyzed by certain complex metal salts in solution This may be described as homogeneous catalysis and, compared to heterogeneous catalysis, is a relatively new development in the area of hydrogenation reactions Rhodium and ruthenium salts appear to be
Heterogeneous Catalysts A Brief Recount Of The Reasons And The Justification That S Support Theoretical Simulations By Jesus M Garcia Figueroa Uprm Department Of Chemical Engineering
What is homogeneous catalyst with example
What is homogeneous catalyst with example- A catalyst is a compound used to help a reaction occur faster by lowering the activation energy There are two types of catalysts, homogeneous and heterogeneous A homogeneous catalyst is aFor example hydrolysis of sugar in the presence of sulphuric acid Heterogeneous Catalysis Heterogeneous catalysis of chemical reactions is a process where the reactants involved in the reaction and the catalyst are in different phases For example reaction of hydrogen and nitrogen in the presence of finely divided iron to form ammonia




Combining Homogeneous And Heterogeneous Catalysis Feature Chemistry World
Heterogeneous catalysts on which chemical reactions occur have been widely used in all aspects of chemical industries 1–3 Compared to precious metals used as catalysts, semiconductors are low cost and have widely tunable chemical and physical properties and thus have been intensively studied as heterogeneous catalysts in many reactions 1–4 The common approach toHomogeneous catalysts are those which exist in the same phase (gas or liquid) as the reactants, while heterogeneous catalysts are not in the same phase as the reactants Typically, heterogeneous catalysis involves the use of solid catalysts placed in a liquid reaction mixtureBecause singlesite heterogeneous catalysts (SSHCs) offer unrivalled opportunities in the fields of green chemistry, clean technology and sustainable development, and also because they present more scope than other kinds of heterogeneous catalysts to design welldefined, atomically characterized, catalytically active centres, they have taken on great significance in the general
Homogeneous catalysts are those which exist in the same phase (gas or liquid ) as the reactants, while heterogeneous catalysts are not in the same phase as the reactants Typically, heterogeneous catalysis involves the use of solid catalysts placed in a liquid reaction mixtureAn advantage of homogeneous catalysis is there is a very high degree of interaction between the catalyst and reactant molecules due to both being in the same phase (as opposed to heterogeneous catalysis) A disadvantage is the homogeneous catalyst is often irrecoverable after the reaction has run to completion In heterogeneous catalysis, the reaction starts at the surface of the solid catalyst and so it is also known as surface catalysis Example Manufacture of H 2 SO 4 by contact process involves oxidation of SO 2 into SO 3 in presence of V 2 O 5 (solid) as catalyst
Catalysts have been identified as homogenous and heterogeneous catalysts Among these catalysts, homogeneous catalysts have been preferred by many researchers for the reason that more biodiesel can be produced relatively at faster rate These homogeneous catalysts are available both in liquid and solid form at ambient conditions• The 1dimensional pseudo homogeneous model without dispersion • Good experimental design to achieve intrinsic information Beyond the scope of the lecture are 1 More complex reactor models (radial dimension) 2 Calculations accounting for transfer limitations 3 Details on parameter estimation in heterogeneous catalysis 4The opposite of adsorption is commonly known as desorption It refers to the breaking apart of the adsorbents and the adsorbate In the chemical reactions that are induced by heterogeneous catalysis, the reactants are the adsorbate and the heterogeneous catalyst is



Heterogeneous And Homogeneous Catalysis For The Hydrogenation Of Carboxylic Acid Derivatives History Advances And Future Directions Chemical Society Reviews Rsc Publishing



Heterogeneous Catalysts A Brief Recount Of The Reasons And The Justification That S Support Theoretical Simulations By Jesus M Garcia Figueroa Uprm Department Of Chemical Engineering
Heterogeneous metal catalysts rather than homogeneous ones are recommended for industrial applications after considering their performance in activity, separation, and recycling 1The diversity of catalysts is large and encompasses all the known categories of catalyst type homogeneous, heterogeneous, and biological This book summarizes the current status in these fields concentrating on rates, kinetics, and reaction mechanisms, but also covers broad topics including modeling, computational simulation, process conceptsIn chemistry, heterogeneous catalysis is catalysis where the phase of catalysts differs from that of the reactants or products The process contrasts with homogeneous catalysis where the reactants, products and catalyst exist in the same phase Phase distinguishes between not only solid, liquid, and gas components, but also immiscible mixtures, or anywhere an interface is present



Q Tbn And9gctrvbbisy7ikjyh35 8msk3jkudkzizuszlwrim5fn3f2i54ygk Usqp Cau




Explain The Difference Between A Homogeneous And Heterogeneous Catalyst Give An Example Of Each Youtube
Examples of Heterogeneous Catalysis and Catalysts – 1 In Haber's process of formation of ammonia, nitrogen and hydrogen are used in gaseous forms while catalyst iron is used in solid form N 2 ( g) 3 H 2 ( g) Fe₍ₛ₎ → Fe₍ₛ₎ 2NH₃ 2In this special issue on 'Homogeneous and heterogeneous catalysis in industry' we report on all aspects of catalysis that are either directly aimed at the development of an industrial process or that can be seen as enabling for such a process In this respect, heterogeneous singlemetalsite catalysts combine features of homogeneous and heterogeneous catalysis Ideally, in metalbased catalysts, any metal atom constitutes a single low




4 Nitrophenol Reduction Catalysed By Au Ag Bimetallic Nanoparticles Supported On Ldh Homogeneous Vs Heterogeneous Catalysis Sciencedirect



Performing Homogeneous Catalytic Ozonation Using Heterogeneous Mn2 Bonded Oxidized Carbon Nanotubes By Self Driven Ph Variation Induced Reversible Desorption And Adsorption Of Mn2 Environmental Science Nano Rsc Publishing
A homogeneous catalyst is a catalyst that is capable of dissolving in solution, because it by definition is in the same phase as the rest of the reactants in the solution Here are the principles of homogeneous catalysts that I see in my textbook (Inorganic Chemistry, Shriver, Atkins, Ch 25) PROS Homogeneous catalysts are effective at being highly selective towardsThe greatest advantage of heterogeneous catalysis is the ease of separation, while the disadvantages are often limited activity and selectivity We report solvents that use tunable phase behavior to achieve homogeneous catalysis with ease of separation Tunable solvents are homogeneous mixtures of wAbstract Surface organometallic chemistry is an area of heterogeneous catalysis which has recently emerged as a result of a comparative analysis of homogeneous and heterogeneous catalysis The chemical industry has often favored heterogeneous catalysis, but the development of better catalysts has been hindered by the presence of numerous kinds




A Review Of The Problem Of Distinguishing True Homogeneous Catalysis From Soluble Or Other Metal Particle Heterogeneous Catalysis Under Reducing Conditions Sciencedirect
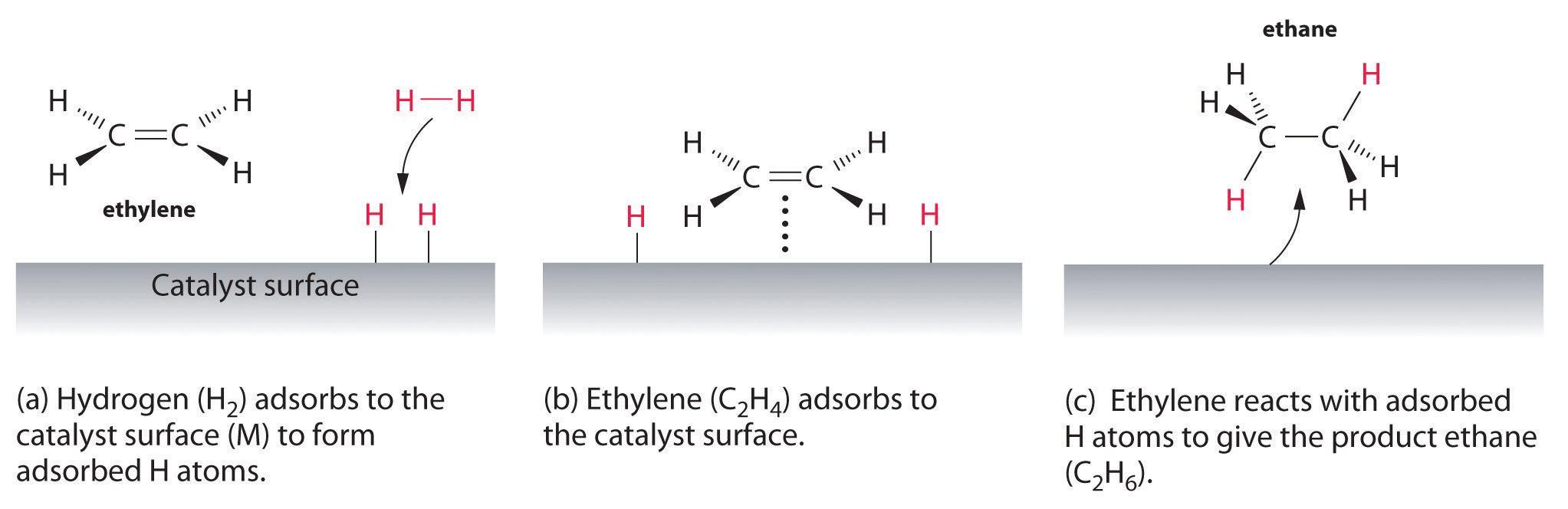



Chapter 13 8 Catalysis Chemistry Libretexts
Catalysis Catalysis Heterogeneous catalysis Many catalytic processes are known in which the catalyst and the reactants are not present in the same phase—that is, state of matter These are known as heterogeneous catalytic reactions They include reactions between gases or liquids or both at the surface of a solid catalyst Since the surface is the place at which the reaction occurs, An attractive strategy to improve the performance of water oxidation catalysts would be to anchor a homogeneous molecular catalyst onto a heterogeneous solid surface to create a hybrid catalyst The idea of this combined system is to take advantage of the individual properties of each of the two catalyst componentsCatalysts Homogeneous & Heterogeneous in a Snap!




1 Homogeneous Vs Heterogeneous Catalysts Download Table
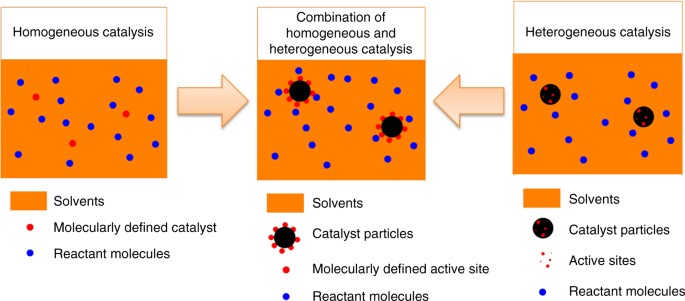



Synthesis Of A Molecularly Defined Single Active Site Heterogeneous Catalyst For Selective Oxidation Of N Heterocycles Nature Communications
Homogeneous and heterogeneous catalysis Activity is the ability of the catalyst to accelerate a chemical reaction The degree can be as high as 100 times in certain reactions A catalytic cycle processes in which the reactant and catalyst undergo several transformations before making theHomogeneous vs heterogeneous catalysis Dr habil Marko Hapke 3 3 Heterogeneous Catalysis Homogeneous Catalysis Catalyst and reactant(s) are in the same phase Catalyst and reactant(s) are in different phases Definitions General features Different reaction phases possible „classic" C−H activation reactions with high catalyst turnover numbers are still very rare in the literature and 10 mol % is a common catalyst loading in this field A representative overview of efficient C−H activation catalysis is presented here to highlight this neglected aspect of catalysis development and inspire future effort towards more




Homogeneous And Heterogeneous Catalysis Gagliardi Group




Research Hermans Research Group
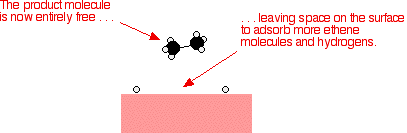
Heterogeneous Catalysis In heterogeneous catalysis, the molecules react at the surface of a solid catalyst The mode of action of a heterogeneous catalyst consists of the following steps Adsorption (or chemisorption) of the reactants on the catalyst surface The reactants diffuse to the surface of the catalyst Homogeneous vs Heterogeneous Catalyst Homogeneous catalysts are catalytic compounds that are in the same phase as the substances which are going into the reaction phase Heterogeneous catalysts are catalytic compounds that are in a different phase from that of the phase of the reaction mixture Phase It is well established that heterogeneous catalysts, in contrast to homogenous catalysts, lose their catalytic activity in the presence of Hg due to the poisoning of their surface 34



Types Of Catalysis Homogeneous Catalysis Heterogeneous Catalysis Positive Catalysis Negative Catalysis Induced Catalysis Acid Base Catalysis




Catalysts Homogeneous Heterogeneous A Level Chemistry Ocr Aqa Edexcel Youtube
Catalysts are conventionally divided into two categories homogeneous and heterogeneous Enzymes, natural biological catalysts, are often included in the former group, but because they share some properties of both but exhibit some very special properties of their own, we will treat them here as a third category Some common examples of catalysis Characterization of Heterogeneous Catalysts 5Steps involved in heterogeneous catalysis 6Catalyst Deactivation 7 Industrial Heterogeneous catalysis 8 Conclusion 3 If the catalyst is present in а different phase than the reactants, it is called а heterogeneous catalyst and this type of catalysis is called heterogeneous catalysis PtNote It is important that you remember the difference between the two terms heterogeneous and homogeneous hetero implies different (as in heterosexual) Heterogeneous catalysis has the catalyst in a different phase from the reactants homo implies the same (as in homosexual) Homogeneous catalysis has the catalyst in the same phase as the reactants



Types Of Catalysis



Heterogeneous Catalysis And Catalyst Recycling All About Drugs
Distinct catalysts are used to accelerate the transesterification reaction, and can be classified into homogeneous and heterogeneous catalysts The homogeneous catalysts used for the alcoholysis reactions may be acid or basic in nature Industrially, basic catalysts are more commonly applied for biodiesel productionCatalysts are generally divided into two types, those that are in the same phase as the reactants (homogeneous catalysts) and those that belong to a different phase (heterogeneous catalysts) The first of the two reactions in this demonstration is an example of a homogeneous catalystThe catalyst is not behaving like a conventional homogeneous molecular catalyst but more like the metallic active sites exploited in heterogeneous catalysts 'We still get singlesite
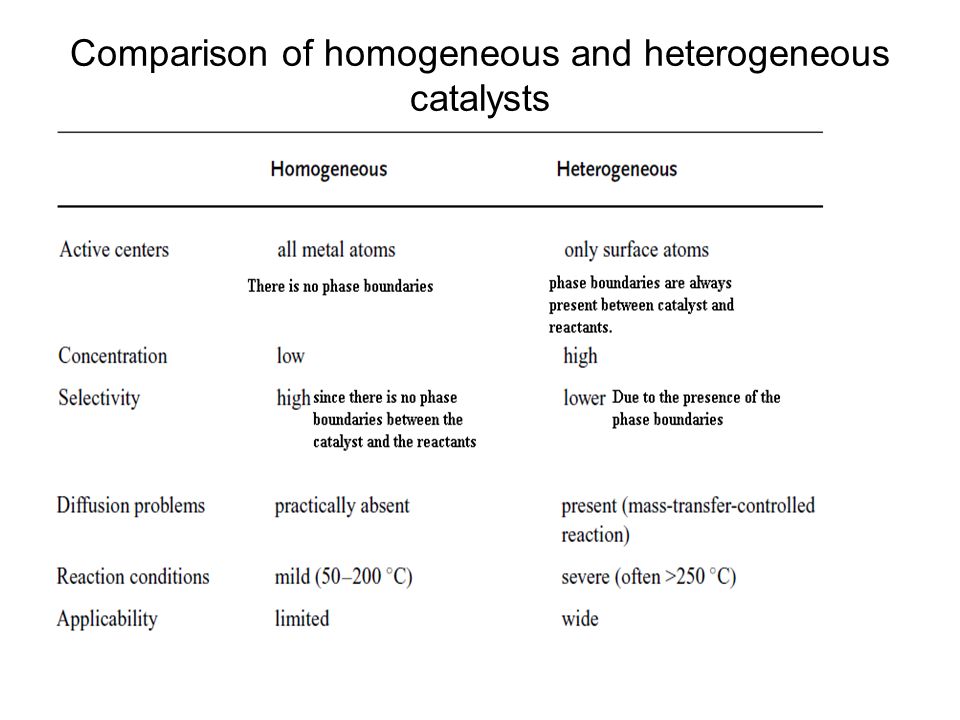



Heterogeneous Catalysis Ppt Video Online Download




Catalyst What Is Catalyst Function Catalyst Function Is Providing An Alternative Reaction Pathway With Lower Activation Energy So That More Reaction Ppt Download
Heterogeneous Heat Transfer might be an issue due to the different heat capacities of reactants and catalystCatalyst Separation Homogeneous The separation of the products from the catalyst is generally expensive, the only exception being in biphasic catalysis Heterogeneous The separation of the products from the catalyst is usuallyThe reactivity of this catalyst was noteworthy, not only because the molecular precursor Re(CtBu)(CHtBu)(CH 2 tBu) 2 is inactive but also because its activity exceeded that of all reported heterogeneous and welldefined homogeneous catalysts known at the time, eg Re 2 O 7 /Al 2 O 3 and Mo(NAr)(CHR)(OtBu F 6) 2 50 The remarkableHomogeneous and Heterogeneous Catalysis We model catalysis, spectroscopy and photochemistry of molecular systems containing transition metals and also catalytic phenomena involving metal or metaloxide clusters attached to a support, like, for example, a



Lab In Hollow Mof Capsules Beyond Integration Eurekalert




Heterogeneous Catalysis Wikipedia
Catalysts and their associated catalytic reactions come in three main types homogeneous catalysts, heterogeneous catalysts and biocatalysts (usually called enzymes) Less common but still important types of catalyst activities include photocatalysis, environmental catalysis and green catalytic processesThe heterogeneous catalysts over the homogenous as it makes the separation e reutilization of heterogeneous catalysts simple and cheap compared to the homogenous catalysts A great variety of homogeneous catalysts are known, ranging from Brønsted and Lewis acids widely used in organic synthesis, metal complexes, metals ions, organometallicHomogeneous catalysis refers to reactions where the catalyst is in the same phase as the reactants, principally in solution In contrast, heterogeneous catalysis describes processes where the catalysts and substrate are in distinct phases, typically solidgas, respectively



Homogeneous Catalyst Chander Jaswal Assistant Professor Chemistry Homogeneous
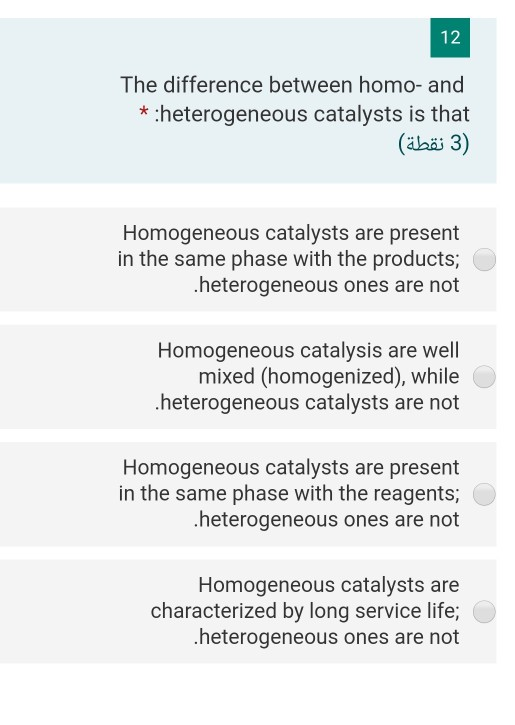



12 The Difference Between Homo And Heterogeneous Chegg Com
This field, "semiheterogeneous catalysis", is at the frontier between homogeneous and heterogeneous catalysis, and progress has been made in the efficiency and selectivity of reactions and recovery and recyclability of the catalytic materialsDifference Between Homogeneous Catalysis and Heterogeneous Catalysis Video Lecture from Surface Chemistry Chapter of Chemistry Class 11 for HSC, IIT JEE, CBSHomogeneous and heterogeneous catalysis With the use of catalysis in chemistry, we can realise reactions under milder conditions and obtain a maximum yield of the desired product Wageningen Food & Biobased Research develops new, catalytic processes for the conversion of biomass into highquality chemicals




Chemical Kinetics Made Easy Homogeneous And Heterogeneous Catalysts Episode 13 Youtube




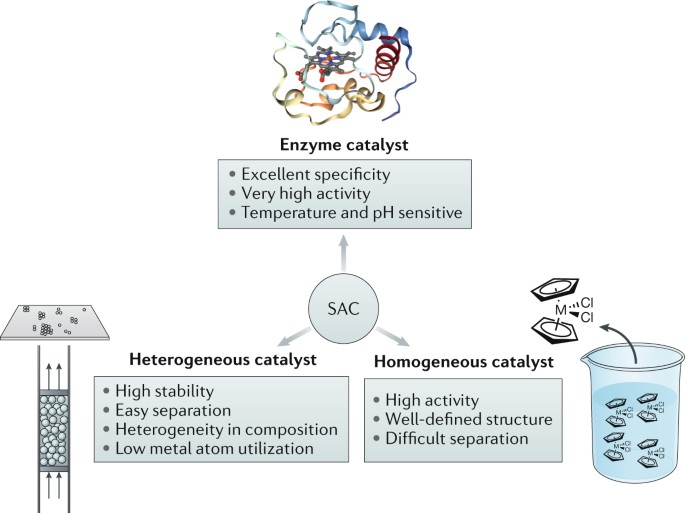
Single Atom Catalyst Based On Homogeneous Catalysis Prototype For Co2 Transformation
Unlock the full Alevel Chemistry course at http//bitly/2ZDDPTi created by Ella Buluwela, Chemistry exper




Heterogeneous Catalyst Goes Enzymatic
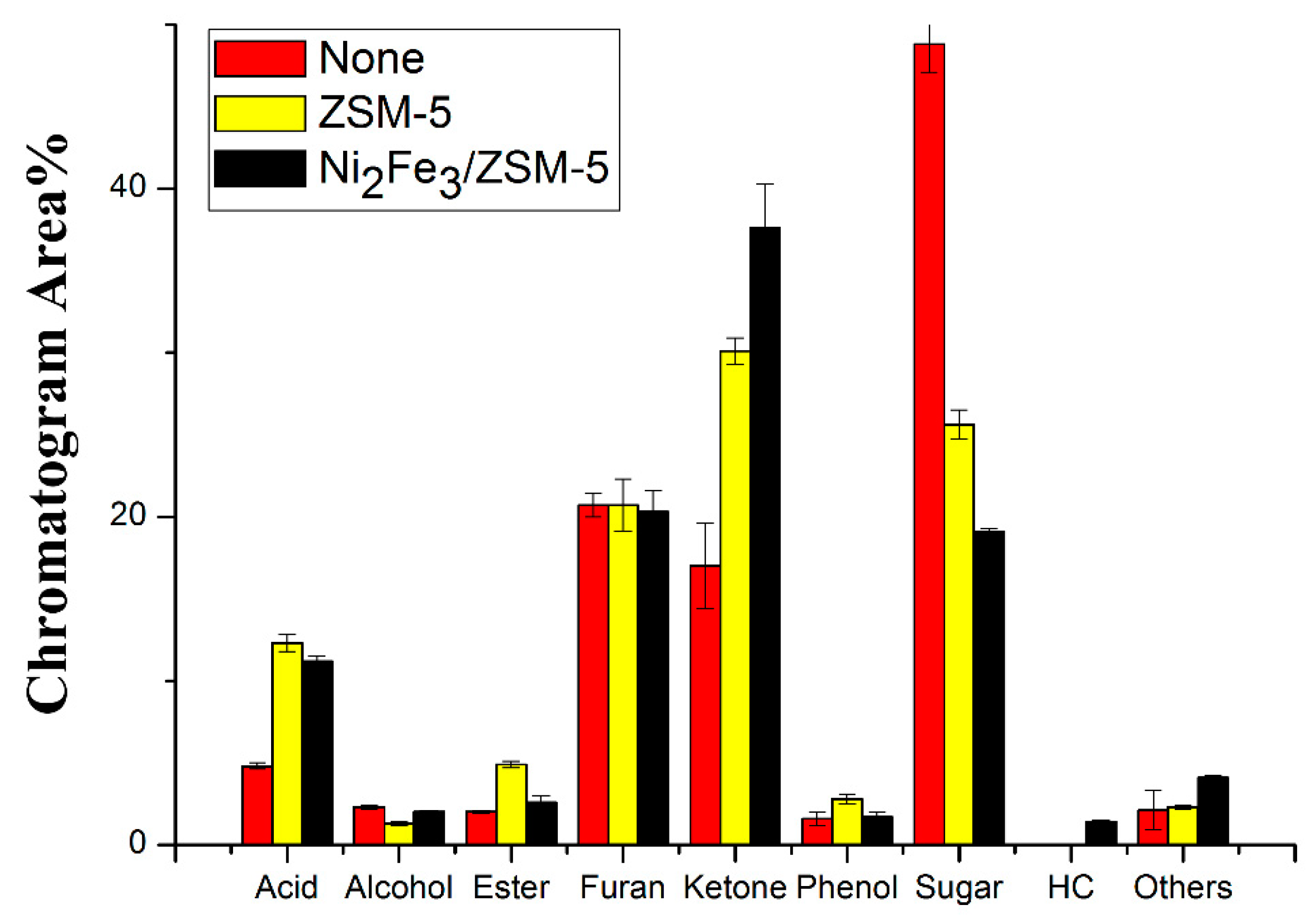



Catalysts Free Full Text Homogeneous And Heterogeneous Catalysis Impact On Pyrolyzed Cellulose To Produce Bio Oil Html




Transition Metals Compounds Acting As Catalysis Catalytic Theory Practice Examples Of Homogeneous Ctalysts Heterogeneous Catalysis Gce As Ib A Level Inorganic Chemistry Revision Notes



Pubs Acs Org Doi Pdf 10 1021 Bk 13 1132 Ch001




Catalysis Boundless Chemistry



Industrial Catalyst Lessons Blendspace



Heterogeneous Catalysis All About Drugs




Combining Homogeneous And Heterogeneous Catalysis Feature Chemistry World



Homogeneous Vs Heterogeneous Catalysts Basic Introduction Youtube




Relationship Among Sacs Homogeneous Catalysts And Heterogeneous Catalysts Download Scientific Diagram



Homogeneous Catalysis Introduction Ppt Video Online Download




Heterogeneous Catalysis Wikipedia



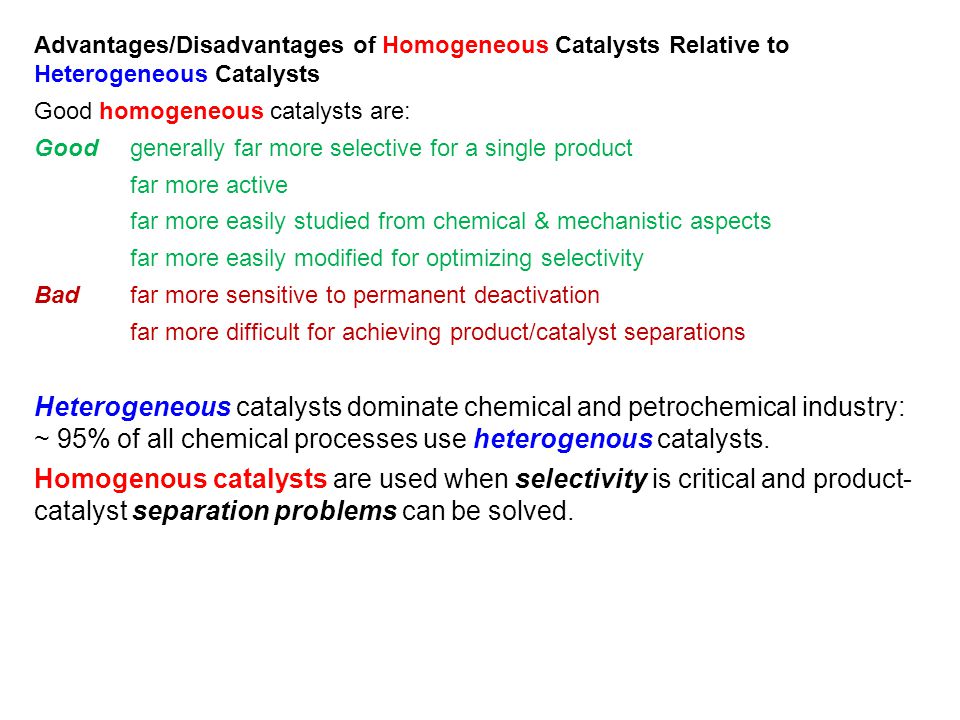
1




Homogeneous And Heterogeneous Catalysis W3spoint



Nitrogen Reduction
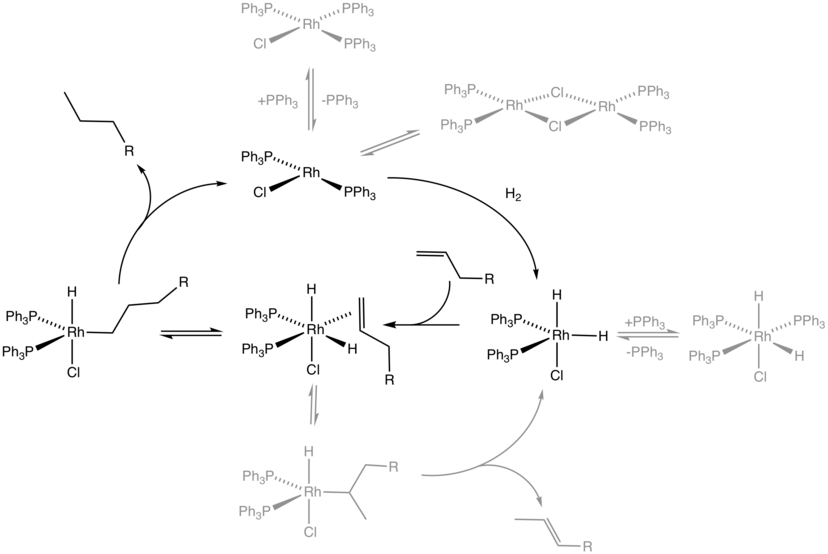



Homogeneous Catalysis Wikiwand




1 Homogeneous Vs Heterogeneous Catalysts Download Table
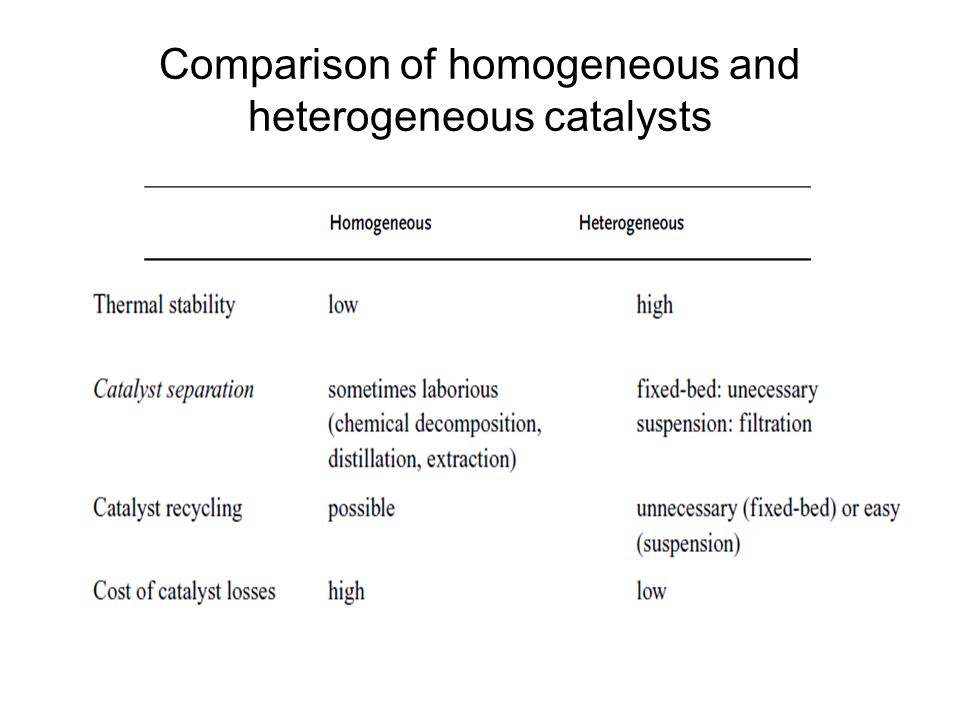



Heterogeneous Catalysis Ppt Video Online Download




Pdf Encyclopedia Of Life Support Systems Eolss Homogeneous And Heterogeneous Catalysis Semantic Scholar



Http Www F U Tokyo Ac Jp Kanai Seminar Pdf Lit Lu D2 Pdf
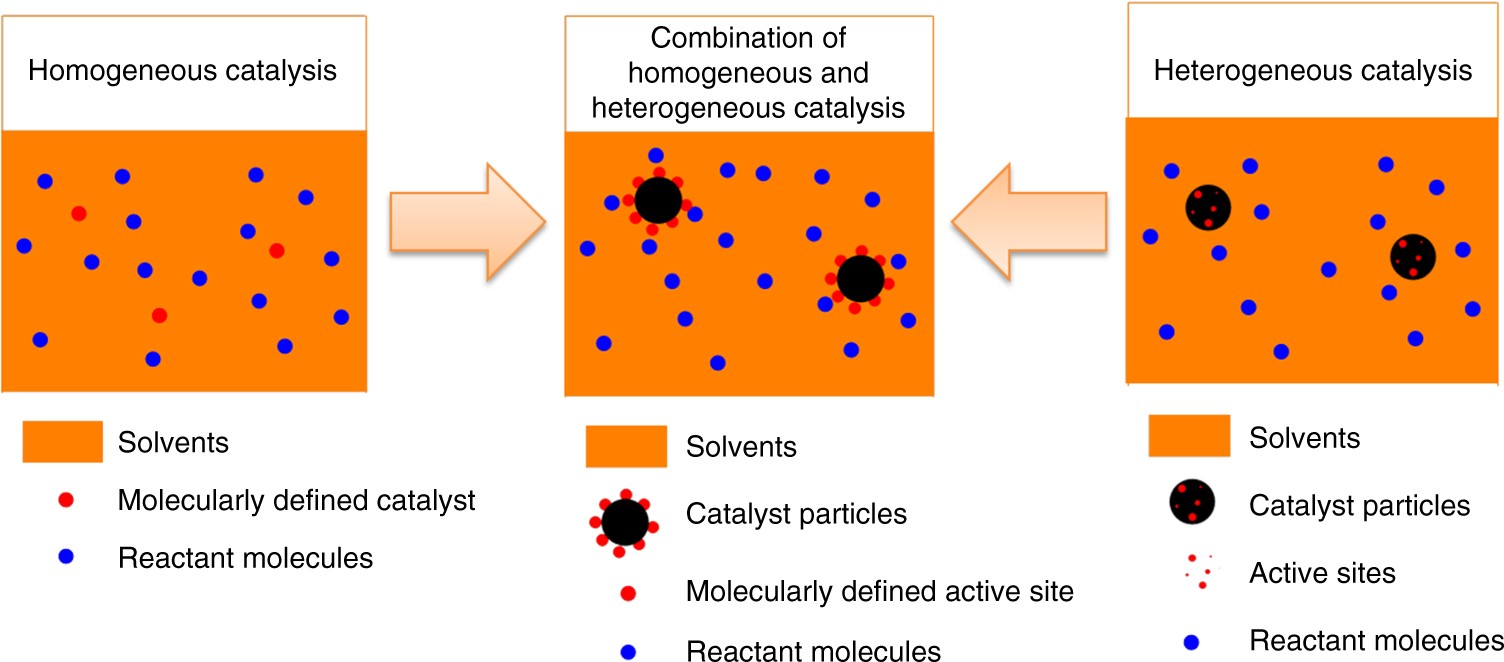



Synthesis Of A Molecularly Defined Single Active Site Heterogeneous Catalyst For Selective Oxidation Of N Heterocycles Nature Communications




Design And Use Of Nanostructured Single Site Heterogeneous Catalysts For The Selective Transformation Of Fine Chemicals Topic Of Research Paper In Chemical Sciences Download Scholarly Article Pdf And Read For Free On
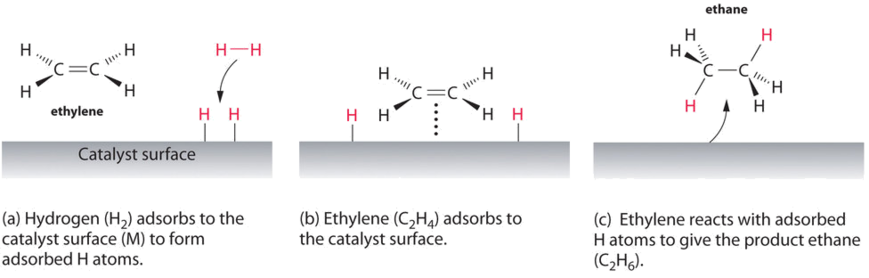



14 7 Catalysis Chemistry Libretexts
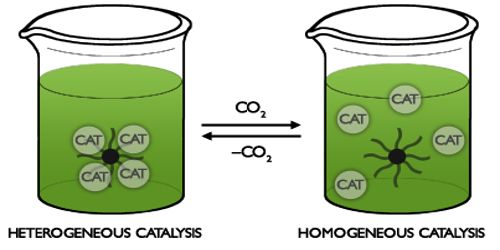



Homogeneous Catalysis Qs Study




Homogeneous Catalysis Wikipedia




Homogeneous Catalyst An Overview Sciencedirect Topics




Comparison Of Biodiesel Production Between Homogeneous And Heterogeneous Base Catalysts Scientific Net




Advantages And Disadvantages Of Homogeneous And Heterogeneous Catalysts Download Scientific Diagram
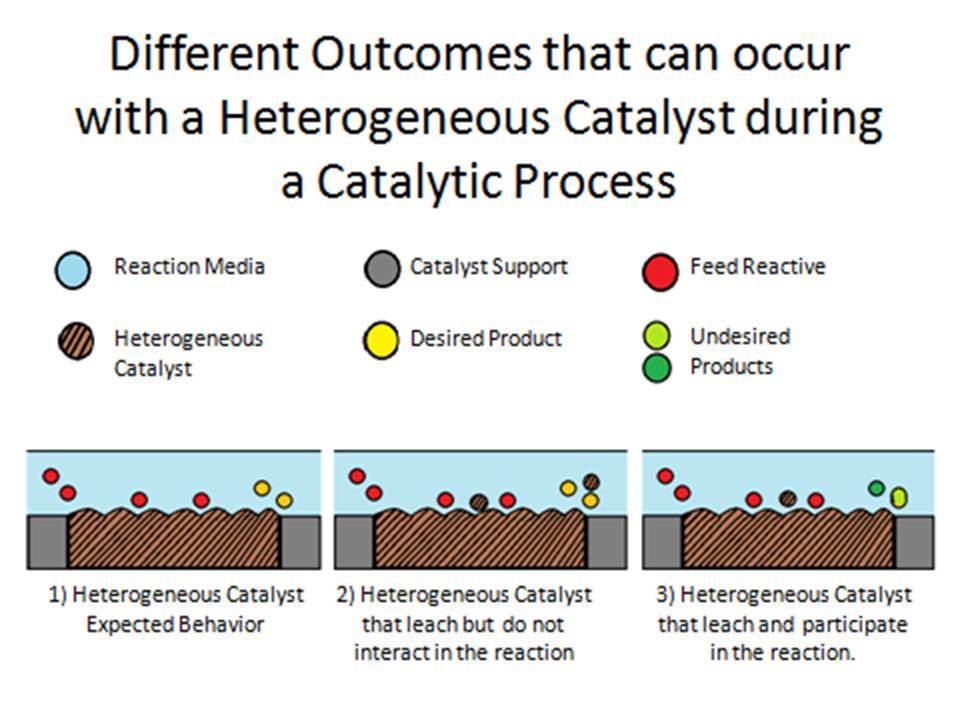



Heterogeneous Catalysts A Brief Recount Of The Reasons And The Justification That S Support Theoretical Simulations By Jesus M Garcia Figueroa Uprm Department Of Chemical Engineering




The Best Of Both Catalytic Worlds Berkeley Lab




Organometallic Catalysis




Heterogeneous Catalysis Flashcards Quizlet



Heterogeneous Catalysis Wikipedia




1 Schematic Diagram Of The Reaction Pathes In Homogeneous Download Scientific Diagram




Figure 26 1 From Homogeneous And Heterogeneous Catalysis Semantic Scholar




Ch3010 A What Are The Advantages And Disadvantages Chegg Com



23 5 Features Of Homogeneous Catalysis A Catalyst



Www Ethz Ch Content Dam Ethz Special Interest Chab Icb Van Bokhoven Group Dam Coursework Catalysis 17 Homogeneous Heterogeoenous Catalysis Mesoporousmaterials Pdf



Question 8 10 Points A What Is The Difference Chegg Com




Pdf Combining The Benefits Of Homogeneous And Heterogeneous Catalysis With Tunable Solvents And Nearcritical Water Semantic Scholar




Heterogeneous Homogeneous Catalysts Video Lesson Transcript Study Com




C 4 1 Compare The Modes Of Action Of Homogeneous And Heterogeneous Catalysts Youtube




Comparative Investigation Of Homogeneous And Heterogeneous Bronsted Base Catalysts For The Isomerization Of Glucose To Fructose In Aqueous Media Sciencedirect



Catalytic Mechanisms Of Hydrogen Evolution With Homogeneous And Heterogeneous Catalysts Energy Environmental Science Rsc Publishing




Chapter 13 8 Catalysis Chemistry Libretexts




Difference Between Homogeneous Catalysis And Heterogeneous Catalysis Surface Chemistry Youtube




Reshaping The Cathodic Catalyst Layer For Anion Exchange Membrane Fuel Cells From Heterogeneous Catalysis To Homogeneous Catalysis Ren 21 Angewandte Chemie Wiley Online Library
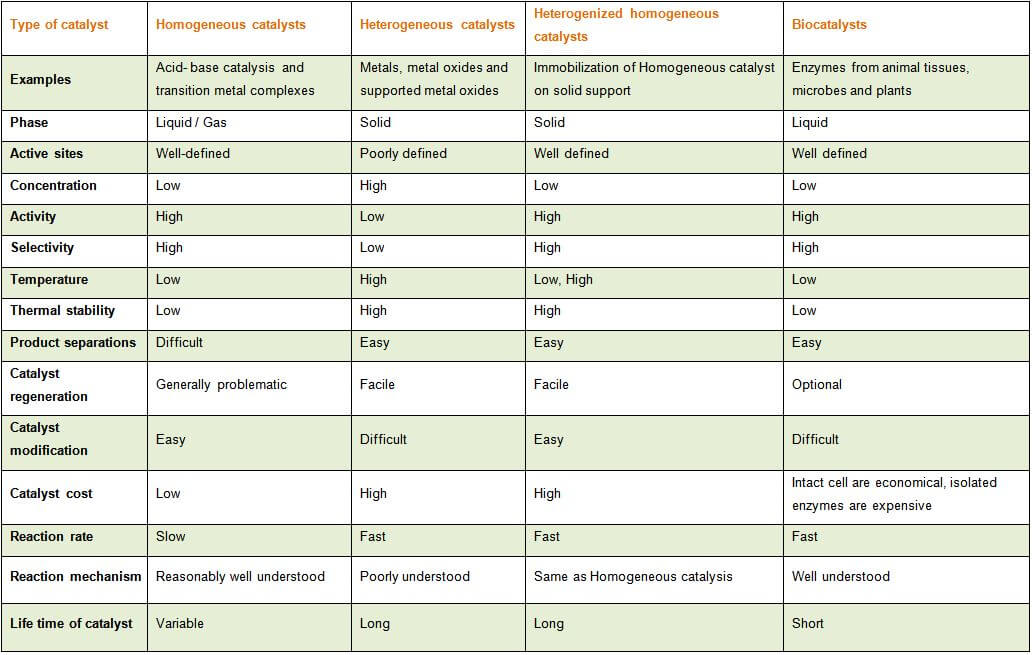



An Overview Of Different Types Of Catalysts Legal Advantage
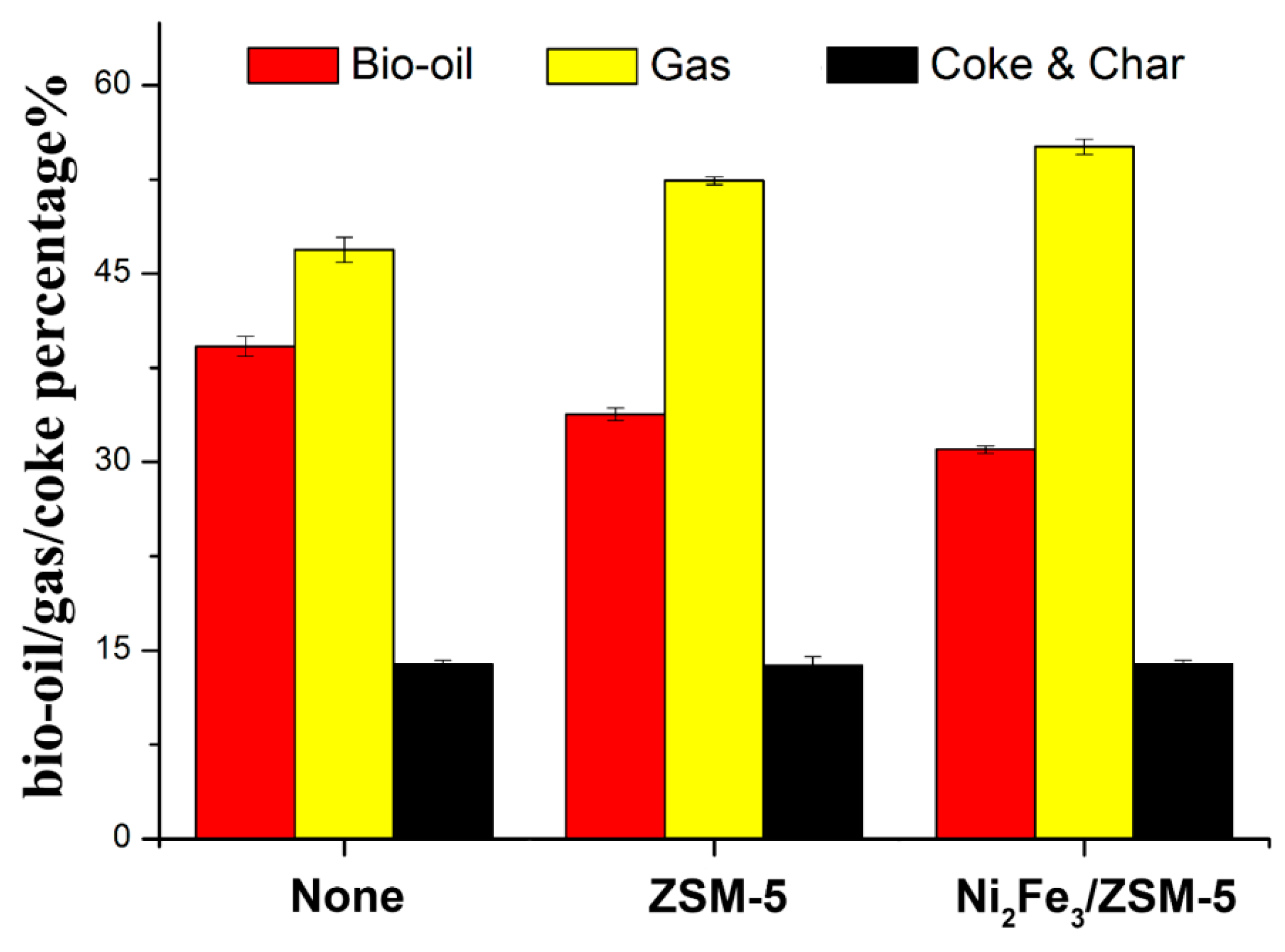



Catalysts Free Full Text Homogeneous And Heterogeneous Catalysis Impact On Pyrolyzed Cellulose To Produce Bio Oil Html




Kinetics Theory Of Catalytic Mechanisms Heterogeneous Catalysis Homogeneous Catalyzed Reaction Examples Advanced A Level Gce Revision Notes
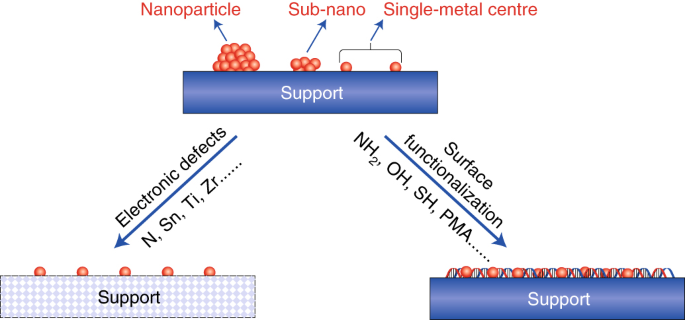



Bridging Homogeneous And Heterogeneous Catalysis By Heterogeneous Single Metal Site Catalysts Nature Catalysis




1 Give Three Reasons Why Heterogeneous Catalysts Are Chegg Com
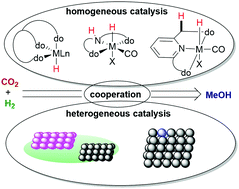



Homogeneous And Heterogeneous Catalysts For Hydrogenation Of Co2 To Methanol Under Mild Conditions Chemical Society Reviews Rsc Publishing
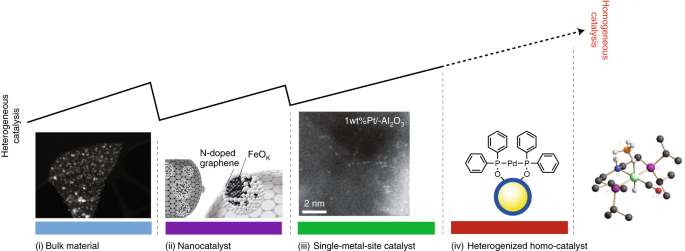



Bridging Homogeneous And Heterogeneous Catalysis By Heterogeneous Single Metal Site Catalysts Nature Catalysis



1




Summarizing Comments On The Discussion And A Prospectus For Urgent Future Action Philosophical Transactions Of The Royal Society A Mathematical Physical And Engineering Sciences
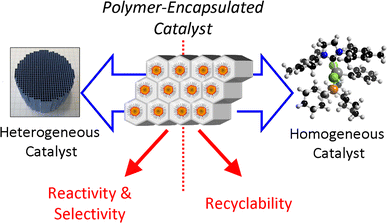



Polymer Encapsulated Metallic Nanoparticles As A Bridge Between Homogeneous And Heterogeneous Catalysis Springerlink




Crossing The Divide Between Homogeneous And Heterogeneous Catalysis In Water Oxidation Pnas




Identity Each Of The Following Catalysts As Homogeneous Or Heterogeneous The Addition Of A Strip Of Brainly Com




Differences Between Homogeneous And Heterogeneous Ef Processes Download Table




What Is The Difference Between Heterogeneous Chegg Com
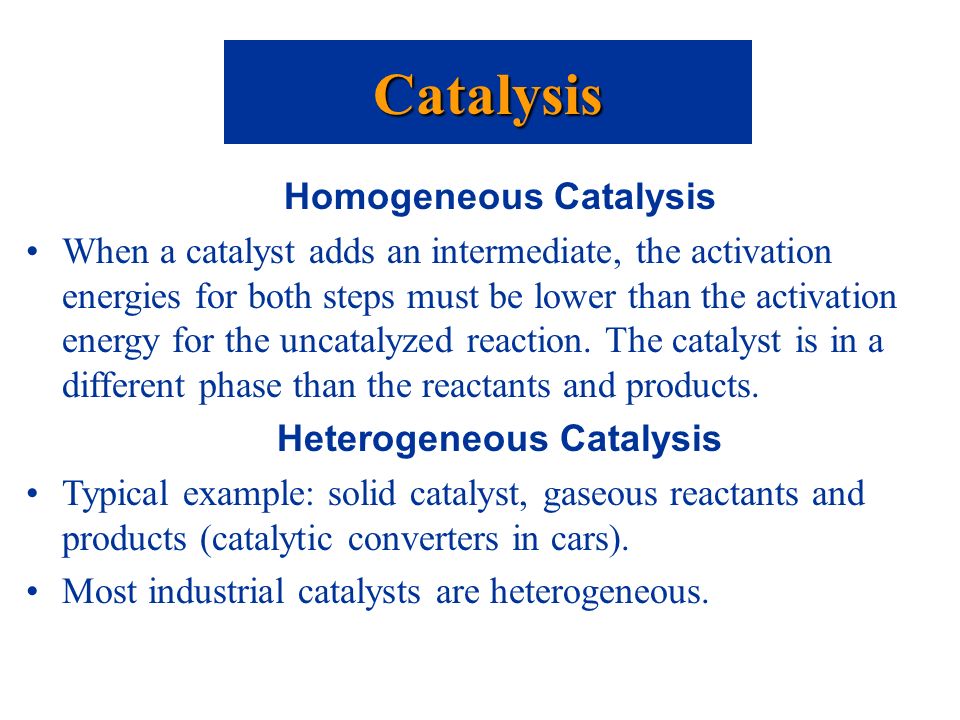



Catalysis Ppt Video Online Download




Catalysis Boundless Chemistry




Asymmetric Heterogeneous Catalysis




Heterogeneous Catalysts A Brief Recount Of The Reasons And The Justification That S Support Theoretical Simulations By Jesus M Garcia Figueroa Uprm Department Of Chemical Engineering



Combining Homogeneous And Heterogeneous Catalysis Feature Chemistry World



Heterogeneous And Homogeneous Catalysis For The Hydrogenation Of Carboxylic Acid Derivatives History Advances And Future Directions Chemical Society Reviews Rsc Publishing



How Can Heterogeneous Catalysts Differ From Homogeneous Catalysts Quora



Homogeneous Versus Heterogeneous Catalysis Download Scientific Diagram




Comparison Of Homogeneous And Heterogeneous Catalysts Download Scientific Diagram




Difference Between Catalytic And Non Catalytic Reaction Compare The Difference Between Similar Terms



Q Tbn And9gctrvbbisy7ikjyh35 8msk3jkudkzizuszlwrim5fn3f2i54ygk Usqp Cau



Http Www Ijetsr Com Images Short Pdf 1496 1500 Ieteb410 Ijetsr Pdf




Single Atom Catalysis Bridging The Homo And Heterogeneous Catalysis Sciencedirect




26 Homogeneous And Heterogeneous Catalysis Chapter




What Are Some Examples Of Homogeneous Catalysis Quora



Bridging Homogeneous And Heterogeneous Catalysis By Heterogeneous Single Metal Site Catalysts Nature Catalysis




Homogeneous Catalysis Catalysis Heterogeneous Catalysis



Types Of Catalysis



Heterogeneous Single Atom Catalysis Nature Reviews Chemistry
コメント
コメントを投稿